
Henry Quach
Optical EngineerFluorescence and Phosphorescence
Like other aspects of product design, optical properties influence a product's functionality and appeal to consumers. Subtle brightness enhancements are engineered into everyday objects, such as laundry detergent, mail envelopes, and regular white paper. We'll get to the details in a bit, but to start, here's simple example about the importance of illumination. Here is a lightbulb that I printed. It's hollow, printed with 0% infill, and weighs about 20 grams. If one were to ask , 'What color is the lightbulb?' , what would you say?


You can drag the slider around see the differences with and without external lighting. The bulb is plausibly off-white to the human eye under visible lighting, but clearly glows green in the dark. The glowing of the plastic masterial is only apparent when it isn't out-competed by the externally-powered white light present from the room.
Color and Light (Huge Tangent; Can Skip Past for Cool Stuff I actually made)
Color is inherently perception-based. Color not only depends on material properties, but also illumination in the environment and the observer. Our vision is possible because radiating energy from object surfaces, whether intrinsically generated by the object or reflecting from an external source, meet our eye. Human eyes are sensitive to the energy content of electromagnetic radiation, which we perceive as color. 'Blue' light represents electromagnetic radiation with a wavelength around 450 nm, while 'red' light represents that with around 680 nm. This mapping of wavelength to our perception is a consequence of evolution.
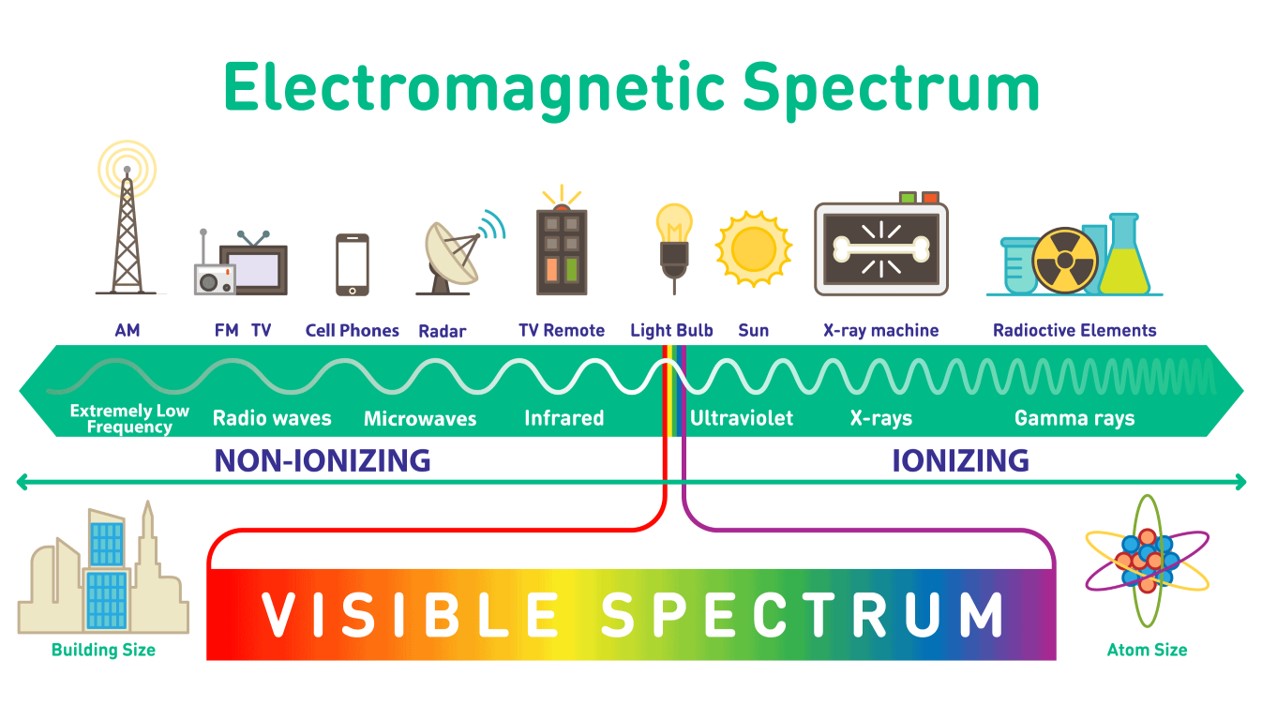 P/C:Defender Shield: What is the EM Spectrum?
Some extra fun facts are that AM radio waves are around 300 meters, FM radio waves are around 3 meters, Wifi and Bluetooth and microwave ovens share wavelengths of around 12.5 cm, and X-Rays have wavelengths from 10 nm to 0.1 nm.
P/C:Defender Shield: What is the EM Spectrum?
Some extra fun facts are that AM radio waves are around 300 meters, FM radio waves are around 3 meters, Wifi and Bluetooth and microwave ovens share wavelengths of around 12.5 cm, and X-Rays have wavelengths from 10 nm to 0.1 nm.
To navigate the once-submerged terraria that revolves arounds the Sun, our ancestors evolved a sensitivity the portion of the electromagnetic spectrum that propagates best through water, 400-700 nm. The Sun is approximately 5778 K and therefore emits from the ultraviolet (<400 nm) to the infrared (>800 nm) due to the laws of Blackbody Radiation; also, the spectral speak occurs around 500 nm. Accordingly, the cones and rods within our eyes can detect radiant energy between 380 - 740 nm, so the wavelength range 400-700 nm is anthropocentrically named the 'visible light' spectrum.
This is but a slice of the full electromagnetic spectrum. EM radiation of all scales (well, mostly ultraviolet and up) surrounds us at all times - flowing through space, bouncing off objects, and going directly through them. Depending on the wavelength of the radiation, light can interact with materials in completely different ways. Thus, materials have spectral properties.
A basic example of spectral material responsivity is apparent in these plastic dragon eggs I printed. All of them were made of the same base PLA plastic (which is naturally translucent!), but had different colorants added to them. Colorants are chemicals that absorb all light except the color that we perceive them to be. For example, the blue dragon egg is illuminated by white light, but the blue colorant absorbs all light except blue, which is reflected back into the room and makes its way to our eyes. All other spectral components of the white light is absorbed, a conversion to heat.
Anyway, the reflection mechanism from external sources is the dominant mechanism by which light from everyday object surfaces get to our eyes. This has been a really long tangent, but I hope this has been somewhat interesting to you.
Glow-in-the-Dark Stuff

In this image, I did some quick SolidWorks modeling to create monolithic cards. Most white PLA is naturally fluorescent due to optical brightness additives in the added white pigment. Glow in the dark filament is a specialty material. The one above is translucent PLA plastic doped with powderized strontium aluminate . SrAl2O4 is a phosphor (which are not necessarily chemically composed of phosphorous).
Objects that intrinsically generate light themselves but not from being hot (this is called incandescence; equivalently, blackbody radiation) are called luminescent objects. Some examples are in the image above, illuminated by an ultraviolet tube light. For all of the build up, all that you need to know is that the higher-energy ultraviolet light emits from the tube and is absorbed by both materials - the typical white filament and the GITD filament. Instead of being absorbed and completely converted to heat as usual, some amount of that absorbed energy is re-emitted. However, from the conservation of energy, we know that the energy of the re-emitted light cannot be the same as what came in - the emitting light has to be of lower energy (or equivalently, of higher wavelength).


In this GITD OSC logo object, a 1100 lumen white-light flashlight was shined on its surface for about 20 seconds. The violet, blue, and some of the blue-green wavelengths were absorbed and some of its energy was re-emitted as green light. Red, yellow, and wavelengths (with less energy than the emitting green light) were completely absorbed as heat with no re-emission. The green wavelength emission is a consquence of the quantum mechanical properties of material. Nature favors lower energy states, so the energy drips out in the form of luminescence during a quantum electronic transition at some rate determined by the material.
Phosphorescence
The process described above is called phosphorescence. Phosphorescence is what we associate glow in the dark stars and moons that are used to decorate childrens bedrooms or nurseries. All you need to know is that this is a two step process by which the energy decay to a stable level is relatively slow (as low as thousandths of seconds).
GITD filaments typically contain some metal particulate that is mixed into the plastic. For my filament, strontium aluminate powder was thrown into a hopper along with the thermoplastic beads, which was strung into spools. The most common particulates are Zinc Sulfide and Strontium Aluminate. If you are familiar with powdered metals, you will know that they are in fact used in polishing, and therefore grind the soft brass away. I included the next section as another tangent on the consquences of using this filament on my printer.
According to all that I've said, it makes sense that three seconds of the UV light exposure made the filament glow far brighter than 20 seconds of the 1100 lumen flashlight. It was really fun to see the difference between the two lighting scenarios. There are definitely other transition metals that make good for good phosphores, but the reason why the Zinc Sulfide and Strontium Aluminate are the most popular is being they are cheaper to process, non-toxic (also part of being cheaper), and green wavelengths are what human eyes are most responsive to.
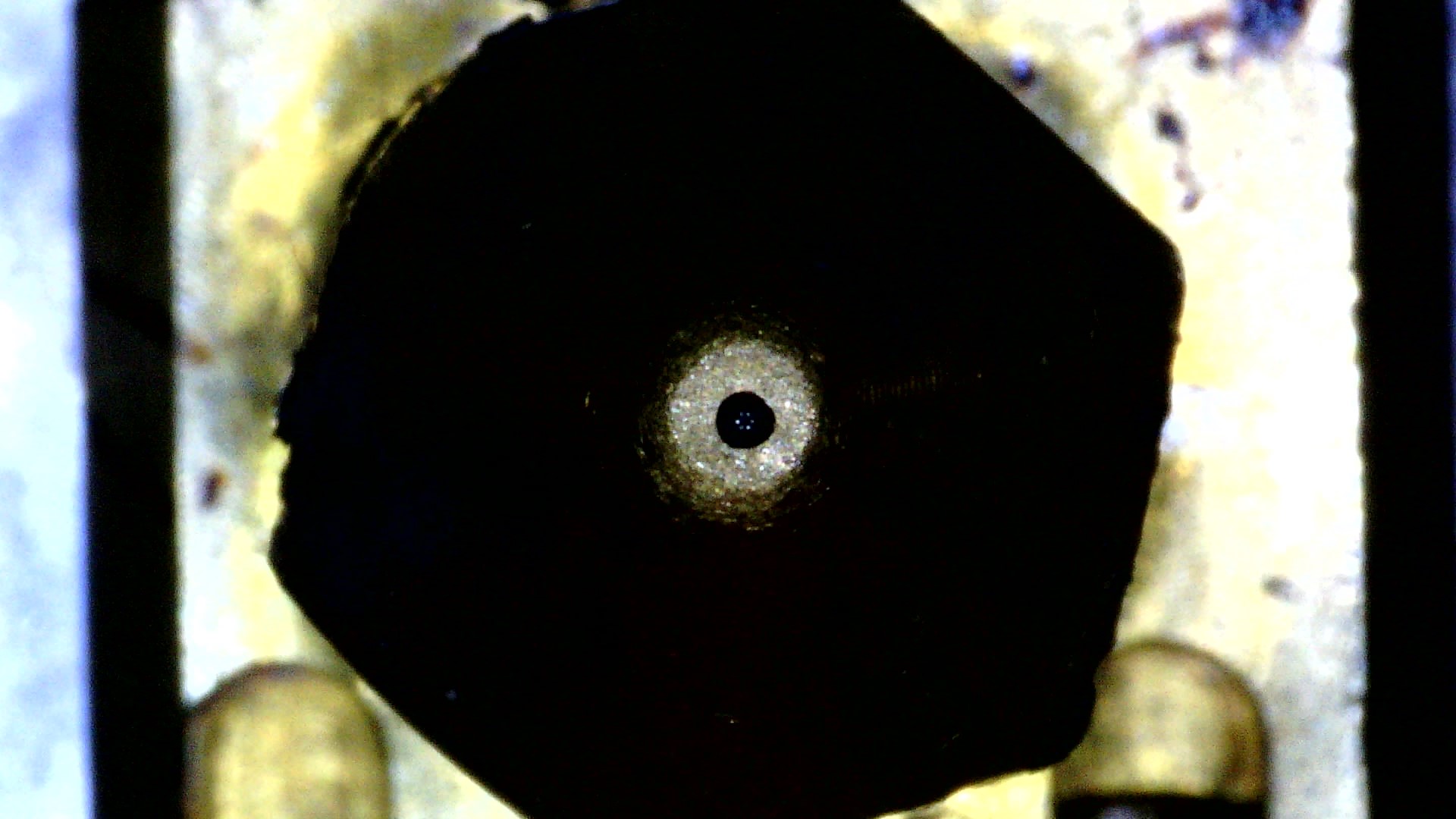
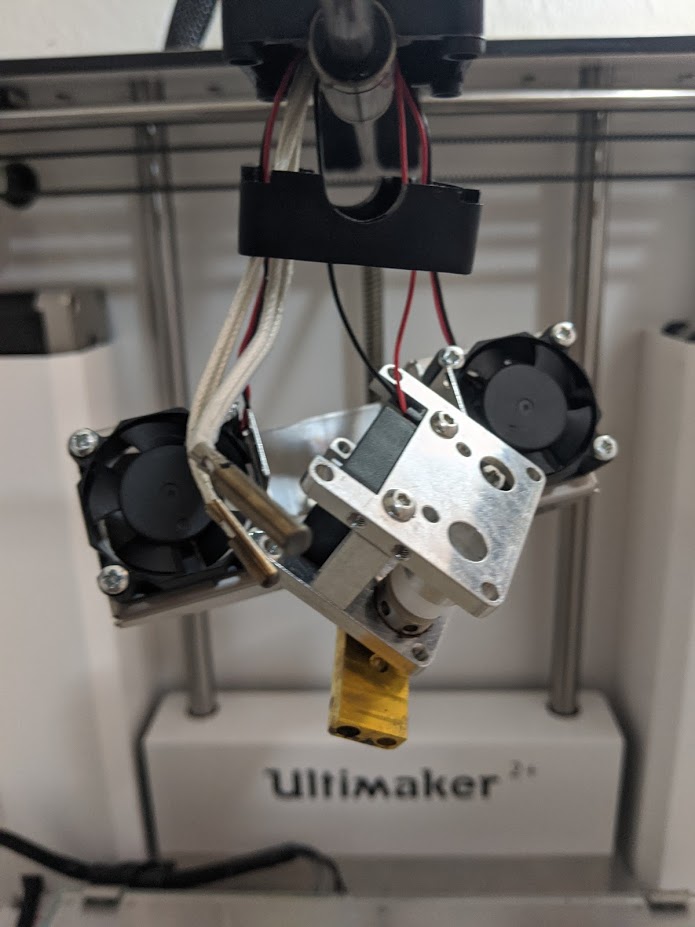
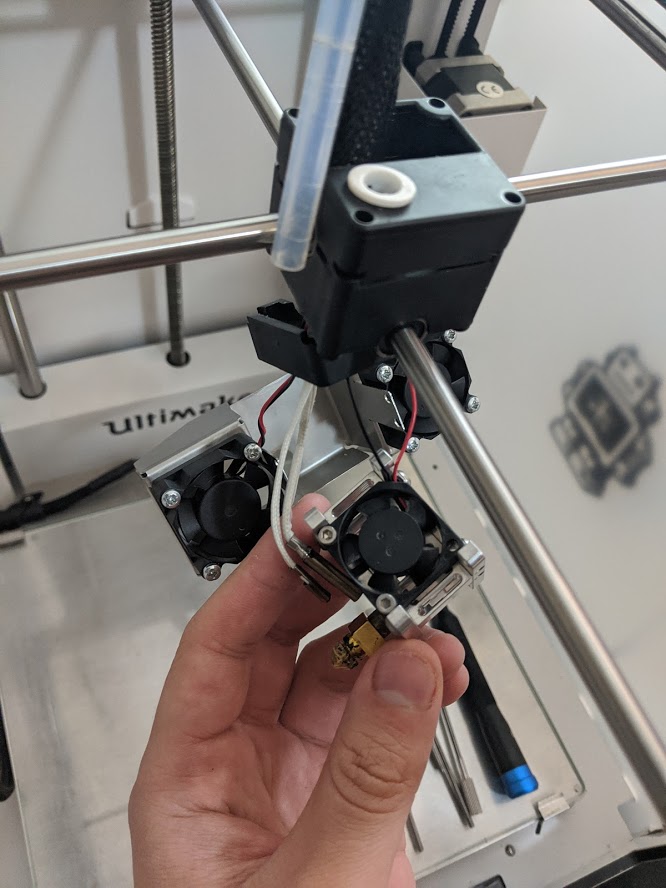
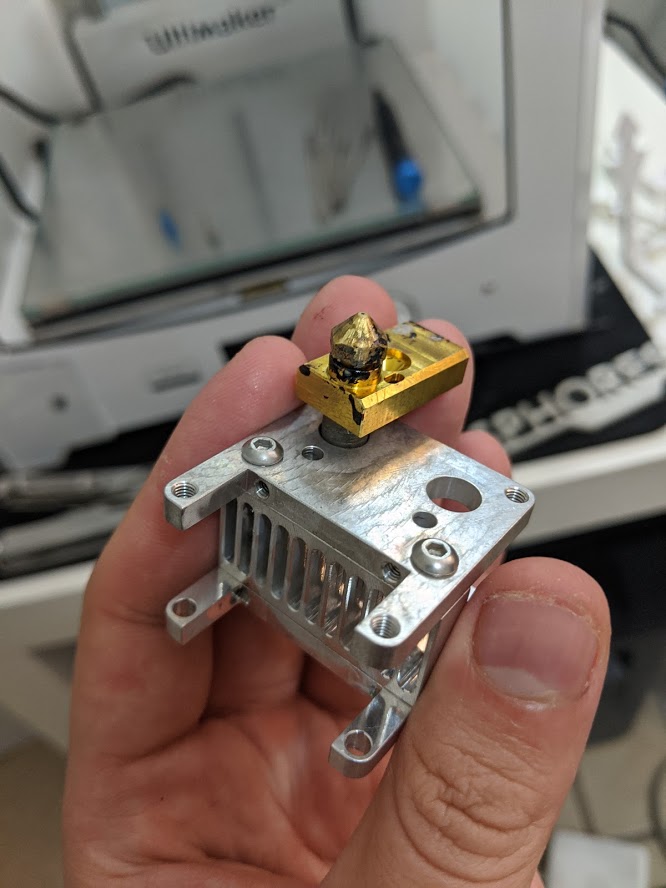
Print Nozzle Erosion
Nozzle abrasion was rampant. I'm rather lazy at the moment, but as a summary before I come back to formalize this page... Printing only 250 grams of the material, I determined the nozzle had worn from a nominaml 0.40 mm to at least 0.48. This is significant as the back stepper motor needs to know it has to push in more filament to get correct extrusion. Without modification to slicing settings, the 3D printer underextrudes. I measured the effects using a USB microscope and used ImageJ to reference the diameter of a typical electroncis pin header, whose width was measured with a caliper. This worked shockingly well.




Some other fun stuff I was able to print was the classic cute octopus waving high, as well as a Kodama from Princess Mononoke. Using the brilliant design of the creator (need to find the source again) as he intended, I slipped some rice into its hollow head it can make the classic rattling sounds. I also did a vector trace of THE SCARY DOOR from Futurama - I'll upload that to Thingiverse later in the summer when I have some extra time too.




Ultimately, I replaced the nozzle so I could maintain the degree of accuracy I wanted for functional prints. I ended up buying a socket head to remove the print, after thinking I could not apply enough force (even when the print head was taken apart!), but actually I had been turning the wrong way. Whoops.


Fluorescence and Everyday White Objects
Despite all of this rambling, I haven't even gotten to the coolest part of luminescent stuff yet. Fluorescence is a one step decay process, which is, again, a result of quantum mechanical material properties. During absorption, some energy from the incident light is automatically lost to the lattice, but the rest is available for near-instantaneous emission. Ultraviolet light (200-400 nm) is not visible to our human eyes, but it is extra energy that is commonly available in our environments. This is from common incandescant sources such as the sun and tungsten lightbulbs.
Everyday white materials like cotton and wood pulp (for paper) are naturally slightly tinged towards yellow, but we can offset this effect by addiing optical brighteners to them. Optical Brightening Agents (OBA's) are materials that absorb ultraviolet light and re-emit some of that as blue. If you add this phosphor to cotton or wood pulp, the color coming from them balances out towards something more perceptibly white. These have gotten naturally cheaper over time, so they're ubiquitous among most of our copy paper, cotton clothing, and even furniture paints. I'll put some more pictures about this later.
Fluorescence doesn't have to only be for emitting blue light. The emitting wavelength again depends on what's allowable for the one-step electronic transition, and could be yellow, green, red, etc. For example, special fluorescent dyes are used to tag postal mail for identification and cancellation (don't reuse stamps!!!), as well as verifying the legitimacy of currency.
Lastly, there's some cool stuff related to fluoescence in the art world. You'll notice that if you pin a piece of white paper against the wall, it'll get more yellow over time to due exposure to the sun. OBAs have finite lives (although they've gotten better and better over the years), and so artists have to be careful about the mediums they choose. Canvases and paper doped with OBAs cannot be guaranteed to be perceived human eyes in the same way over many years because of the OBA's decay. Some commercial solutions have occurred, such as special frames with glass that filter out the UV (polycarbonate is EXCELLENT for filtering out ultraviolet light and used in rated safety glasses), spectral filters for museum illumination sources, and even spectral filters for flash photography illumination modules. Anyway, that's what I got!